INFECTA strongly believes that well characterized CHIM can accelerate product development in several key areas of vaccine and drug development for infectious diseases.
CHIMs
Controlled Human Infection Models (CHIM) are carefully designed studies in which healthy adult volunteers are exposed to a well-characterised strain of a pathogen under highly regulated conditions.
The purpose is to observe the earliest stages of infection, measure immune responses in real time and, when applicable, test the protective effect of a candidate vaccine or therapy. Every study is built on a rigorous ethical and safety framework that has been refined over decades, and is continually reviewed by independent committees and national regulators.
The difference between CHIM studies and traditional early-phase trials is that the latter often relies on waiting for participants to encounter a pathogen naturally. This uncertainty prolongs timelines, inflates budgets, and leaves critical questions unanswered.
A CHIM study produces clinical, microbiological and immunological data within weeks. Because the timepoint of exposure is known precisely, the resulting datasets are far richer: they include detailed kinetics of infection, correlates of protection, and proof-of-concept efficacy signals that would otherwise be invisible at this stage. Sponsors gain a clear view of whether a product behaves as intended long before they commit to large-scale field trials.
Sponsors who engage early with INFECTA embed CHIM-thinking into their development roadmap from day one. This alignment reduces protocol amendments later, shortens development cycles and brings promising interventions to people who need them sooner. Whether you are seeking a single exploratory study or a multi-pathogen platform, we offer facilities, regulatory support and scientific leadership under one roof.
Our CHIMs support research across a range of respiratory and vector-borne viruses, such as Dengua & Influenza
Our CHIMs support research across a range of respiratory and vector-borne viruses, such as Dengua & Influenza
Our CHIMs support research across a range of respiratory and vector-borne viruses, such as Dengua & Influenza
Our CHIMs support research across a range of respiratory and vector-borne viruses, such as Dengua & Influenza
Our CHIMs support research across a range of respiratory and vector-borne viruses, such as Dengua & Influenza
Our CHIMs support research across a range of respiratory and vector-borne viruses, such as Dengua & Influenza
Our CHIMs support research across a range of respiratory and vector-borne viruses, such as Dengua & Influenza
Our repository of CHIMs including Bordetella Pertussis and Pneumococcus provides a valuable platform
Our repository of CHIMs including Bordetella Pertussis and Pneumococcus provides a valuable platform
Our repository of CHIMs including Bordetella Pertussis and Pneumococcus provides a valuable platform
Our repository of CHIMs including Bordetella Pertussis and Pneumococcus provides a valuable platform
With our CHIMS for parasites like Malaria & Hookworm, you can ensure that your process is sped-up
With our CHIMS for parasites like Malaria & Hookworm, you can ensure that your process is sped-up
With our CHIMS for parasites like Malaria & Hookworm, you can ensure that your process is sped-up
With our CHIMS for parasites like Malaria & Hookworm, you can ensure that your process is sped-up
Comparison
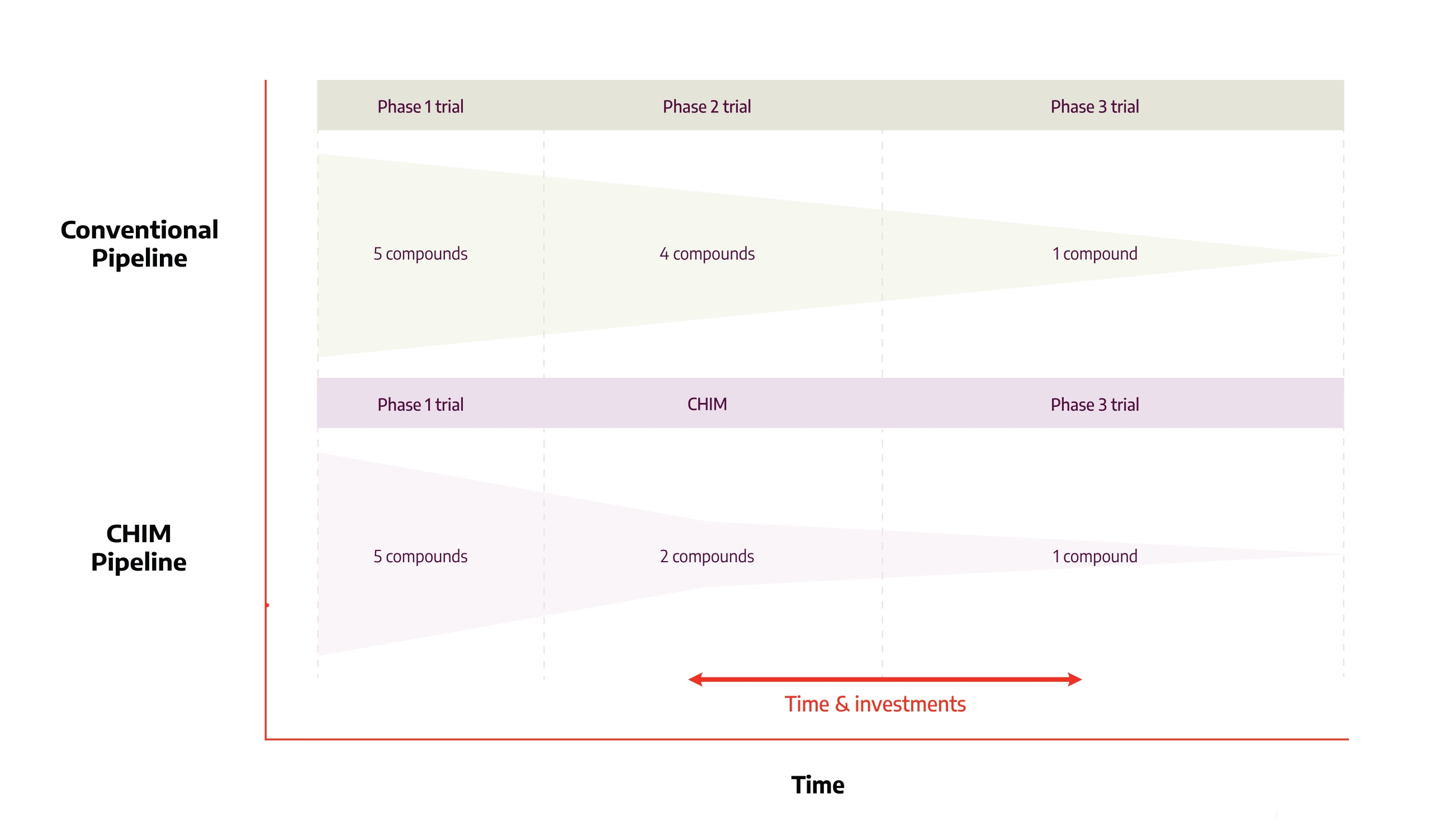
A phase IIb field study designed to demonstrate preliminary efficacy against a seasonal respiratory virus can require thousands of participants across multiple continents and may still be undermined by a mild season with a reduction in the number of cases.
A CHIM study targeting the same respiratory virus typically involves 40-50 volunteers and finishes within 3 months.
The two approaches are complementary rather than competing: a positive CHIM result de-risks the subsequent field program, supports streamlined trial designs and, in some cases, allows for adaptive licensing pathways.
Benefits
Early objective evidence of efficacy changes the conversation with boards, partners and investors. A strong CHIM-dataset demonstrates that a candidate product performs under the most stringent possible test: deliberate exposure. It backs up your immune-response claims with solid data, clarifies the product’s goals, and underpins realistic financial forecasts. This clear evidence lets investors decide sooner and with greater confidence, bringing in capital faster and at a lower cost to your company.
Our expertise
INFECTA maintains the world’s largest portfolio of validated CHIMs, developed through long-standing collaborations with academic leaders and public-health organisations. Our portfolio spans global-health priority pathogens, reflecting a strategy centered on measurable impact rather than short-term commercial trends. Each model has undergone extensive dose-escalation, safety and tested reproducibility, ensuring reliable data outputs that will form a solid foundation for a jump towards licensure.
Our team has run more than 20 CHIM studies since 2005. We understand the practical limits of every model, the regulatory expectations, and the scientific questions that matter most to peer reviewers, policy makers and downstream trial sites. When we advise on a programme, we begin with your product’s mechanism and clinical objectives, map those to an appropriate model and provide transparent guidance on timelines, costs, risk mitigation and data interpretation.
Our team has experience in developing CHIMs for over 5 pathogens for which CHIMs were previously not considered.
If you would like to explore how a CHIM can help you bring your pharmaceutical product to the next level, contact us with the form, or reach out to info@infecta.org